FDA recalls more EYE DROPS due to risk of EYE INFECTION
11/21/2023 / By Olivia Cook
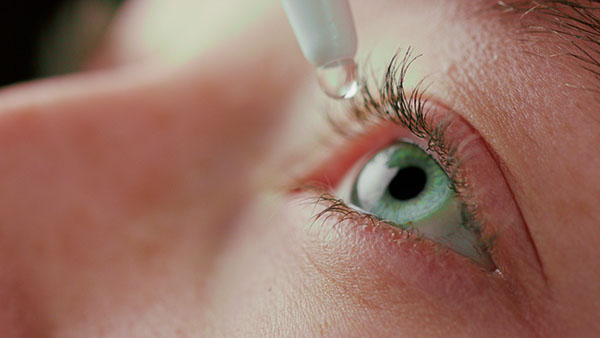
The U.S. Food and Drug Administration (FDA) has issued another recall order for another batch of contaminated eye drops.
CBS News reported that the recall notice issued Nov. 15 arose from potential safety concerns after investigators found “unsanitary conditions” in the facilities where the eye drops were made. This new order came weeks after the regulator also warned consumers against using an earlier batch of products.
The most recent round of recalls involved 28 eye drop products, all coming from lots with expiration dates ranging from November 2023 to September 2025. The products sold on CVS, Rite Aid, Target and Walmart shelves were made by Kilitch Healthcare India Limited – which is headquartered in Mumbai, India.
The agency said the recall of these over-the-counter eye drop products had been prompted by unsanitary conditions in the manufacturing facility and positive bacterial test results from the environmental sampling of critical drug production areas in the facility, according to the investigators’ report.
Last Oct. 30, the FDA issued an alert flagging 26 eye care products from CVS Health, Leader (Cardinal Health), Rugby (Cardinal Health), Rite Aid, Target Up & Up and Velocity Pharma.
Contaminated eye drops linked to fatal outbreak of drug-resistant bacteria
In April, CBS News reported that federal inspection records from Feb. 20 through March 2 showed dozens of issues at Global Pharma Healthcare Pvt. Ltd.’s manufacturing plant in India – ranging from dirty equipment and clothing to missing safeguards and procedures.
The investigation was prompted by a swelling multistate outbreak of a rare and extensively drug-resistant bacteria – Pseudomonas aeruginosa – that was linked to eye drops sold under the brand names EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears and Artificial Ointment.
On March 22, the Centers for Disease Control and Prevention (CDC) update reported that out of 81 patients identified across 18 states with the bacteria, 14 people have lost their vision; four have had their eyeballs removed; and the death toll has climbed to three. (Related: Three people died after using eye drops contaminated with rare bacterial superbug.)
Confirmed infection cases were from California, Colorado, Connecticut, Delaware, Florida, Illinois, North Carolina, Nevada, New Jersey. New Mexico, New York, Ohio, Pennsylvania, South Dakota, Texas, Utah, Washington and Wisconsin.
Patients reported experiencing symptoms, which included blurry vision; clear, green, or yellow discharge from the eyes; eye discomfort or pain; feeling of something in the eye (foreign body sensation); increased sensitivity to light; and redness of the eye or eyelid.
The federal investigators’ report included the following:
- Evidence of poor cleaning procedures throughout the facility – investigators reported surfaces that come into contact with the packaging were “not cleaned, decontaminated, sanitized or sterilized” at all
- A product-filling room that had “soft, unsmooth and cracked sealant, protruding nails and nail holes across its walls”
- Discolored and worn-out booties being used in the company’s “clean rooms”
- Absence of a tracker or monitoring record or have studies that show “how many times plant-issued clothing could be reused by workers”
- Substantial gaps in written procedures and training for workers at the plant
- Unexplained discrepancies wherein an inspector “saw a black-brown greasy deposit” on a part of one of the machines used to fill its products into appropriate receptacles, yet the logs claimed that particular machine had been cleaned just weeks before and had not been used since
- Falling short of double-checking the ingredients it used to produce its products by merely relying on assurances from its suppliers
- Not using appropriate preservatives in its reusable eye drop bottles raise the risk of bacteria growing in already opened bottles
CBS News reported that the March 30 recall of more than 50,000 products labeled Delsam Pharma’s Artificial Ointment was prompted after an analysis by the agency found “unopened tubes of the ointment to be contaminated with bacteria.”
On March 7, also over sterility concerns, eye drop recalls included Pharmedica USA LLC’s “Purely Soothing, 15% MSM” – an anti-inflammatory remedy to help with eye irritation or swelling. Customers were warned to immediately stop using the product because it “may contain bacteria and germs” – which can result in eye infections that could cause blindness.
On March 1, Canadian-based Apotex Corp. voluntarily recalled six lots of its brand of eye drops labeled “Brimonidine Tartrate Ophthalmic Solution, 0.15%,” which is prescribed for patients with open-angle glaucoma or ocular hypertension, “out of an abundance of caution” over some concerns that cracks in some of the units’ caps could compromise the drops’ sterility and lead to infection.
Visit FDA.news for more similar stories.
Watch this news report about the FDA warning the public to stop using 26 types of eye drops immediately.
This video is from the Daily Videos channel on Brighteon.com.
More related stories:
Over 2M at-home COVID-19 test kits recalled due to false positive results.
FDA announces voluntary recall of contaminated eye drops that could blind people.
Sources include:
Submit a correction >>
Tagged Under:
antibiotic-resistant, big government, Big Pharma, CDC, eye drops, eye health, eye infection, FDA, infections, outbreak, pharmaceutical fraud, Product recall, products, Pseudomonas aeruginosa, real investigations, recall notice, superbugs
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 CDC NEWS